Healthy technology
that fits your life
The myMerlinPulse™ app pairs to your compatible Abbott implantable cardiac defibrillator (ICD) or cardiac resynchronization therapy defibrillator (CRT-D) and discreetly goes where you want, when you want— freeing you to live life on your terms.
Download the app today



How to get connected
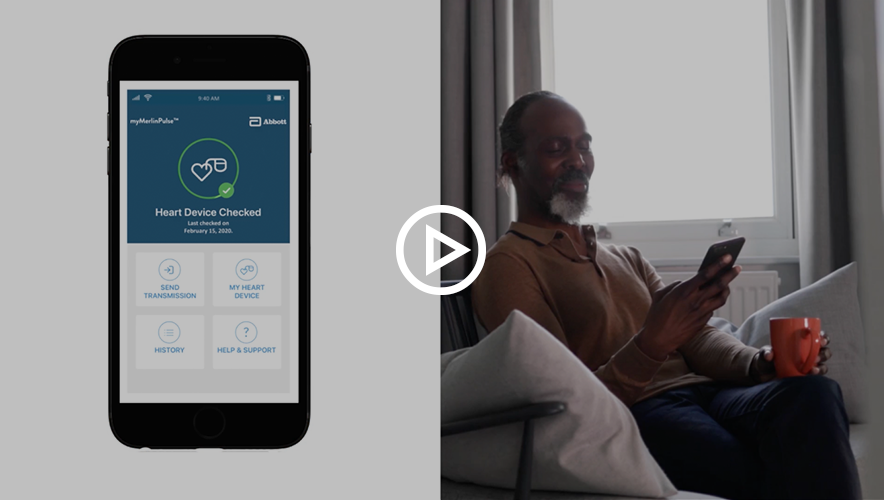
Watch these videos to learn about the myMerlinPulse app and how to connect it to your Abbott device.
SyncUp™
Support and First-Time App Setup
A SyncUP support expert will take you through the set up process for connecting your new device to the myMerlinPulse app. SyncUP support will also be able to answer all your questions about the app and remote monitoring.


Your health
in your hands
The myMerlinPulse app provides the reassurance you need by securely and remotely monitoring your Abbott device. The mobile app uses Bluetooth® wireless technology to transfer your heart rhythm information between the device, the app and your clinic.
Your health
in your hands
The myMerlinPulse app provides the reassurance you need by securely and remotely monitoring your Abbott device. The mobile app uses Bluetooth® wireless technology to transfer your heart rhythm information between the device, the app and your clinic.

Stay engaged in your treatment
Your doctor will set a schedule for collecting information from your Abbott device,
based on your individual health needs.
THE MYMERLINPULSE APP ALLOWS YOU TO:
Common questions
about myMerlinpulse app
Based on how your healthcare provider has set up your app, the app can automatically collect relevant data from your implanted heart device and send it to your clinic. You can also send a transmission from the app, at your clinic’s request.
Inform your clinic of your travel arrangements. Your heart device constantly monitors your heart activity and records cardiac events. If the app detects anything your doctor wants to be aware of, it will send the information to your clinic when you are connected to the internet. Stay connected to the internet (Wi-Fi‡ or cellular data) as often as possible.
Device checks typically occur around 2 a.m. every day. Keep your mobile device near you (within 5 feet or 1.5 meters) when you sleep. If you are not close to your mobile device at that time, the app will retry automatically when you are nearby. Device checks occur automatically. If you open the app and happen to see a “Connect Now” button, you may tap the button to manually complete the check.
Keep your mobile device within 5 feet or 1.5 meters of you. When you go to bed, keep your mobile device on your bedside table next to you.
The first step to a
healthier you
As part of the Abbott family, you can rest assured that we are with you for every step of your healthcare journey. You deserve peace of mind, to go about each day as the individual you are, and the freedom to live life on your terms. Together, we can make that life a reality.

Abbott
15900 Valley View Court Sylmar, CA 91342
Tel: +1 818 362 6822
cardiovascular.abbott
Rx ONLY
BRIEF SUMMARY
This product is intended for use by or under the direction of a Physician. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, potential adverse events and directions for use.
INTENDED USE
The Implantable Cardioverter Defibrillator (ICD) and Cardiac Resynchronization Therapy Defibrillator (CRT-D) devices are intended to provide ventricular antitachycardia pacing and ventricular cardioversion/defibrillation. The CRT-D devices are also intended to resynchronize the right and left ventricles.
The myMerlinPulse™ mobile application is intended for use by people who have an Abbott Medical implanted heart device and access to a mobile device. The app provides remote monitoring capability of the implanted heart device by transmitting information from the patient’s implanted heart device to the patient’s healthcare provider.
INDICATIONS
Indications: The ICD and CRT-D devices are indicated for automated treatment of life-threatening ventricular arrhythmias. CRT-D devices are also indicated to treat symptoms in patients who have congestive heart failure with ventricular dyssynchrony.
In addition, dual chamber ICD and CRT-D devices with the AT/AF detection algorithm are indicated in patients with atrial tachyarrhythmias or those patients who are at significant risk of developing atrial tachyarrhythmias.
MR Conditional ICDs and CRT-Ds are conditionally safe for use in the MRI environment when used in a complete MR Conditional system and according to instructions in the MRI-Ready Systems manual. Scanning under different conditions may result in severe patient injury, death or device malfunction.
The myMerlinPulse™ mobile application is indicated for use by patients with supported Abbott Medical implanted heart devices.
CONTRAINDICATIONS
Contraindications for use of the pulse generator system include ventricular tachyarrhythmias resulting from transient or correctable factors such as drug toxicity, electrolyte imbalance, or acute myocardial infarction.
The myMerlinPulse™ mobile application is contraindicated for use with any implanted medical device other than supported Abbott Medical implanted heart devices.
ADVERSE EVENTS
Possible adverse events associated with the implantation of the pulse generator system include the following: Arrhythmia (for example, accelerated or induced), Bradycardia, Cardiac or venous perforation, Cardiac tamponade, Cardiogenic shock, Death, Discomfort, Embolism, Endocarditis, Erosion, Exacerbation of heart failure, Excessive fibrotic tissue growth, Extracardiac stimulation (phrenic nerve, diaphragm, pectoral muscle), Extrusion, Fluid accumulation within the device pocket, Formation of hematomas, cysts, or seromas, Heart block, Hemorrhage, Hemothorax, Hypersensitivity, including local tissue reaction or allergic reaction, Infection, Keloid formation, Myocardial damage, Nerve damage, Occlusion/Thrombus, Pericardial effusion, Pericarditis, Pneumothorax, Pulmonary edema, Syncope, Thrombosis, Valve damage. Complications reported with direct subclavian venipuncture include pneumothorax, hemothorax, laceration of the subclavian artery, arteriovenous fistula, neural damage, thoracic duct injury, cannulation of other vessels, massive hemorrhage and rarely, death. Among the psychological effects of device implantation are imagined pulsing, depression, dependency, fear of premature battery depletion, device malfunction, inappropriate pulsing, shocking while conscious, or losing pulse capability. Possible adverse device effects include complications due to the following: Abnormal battery depletion, Conductor fracture, Device-programmer communication failure, Elevated or rise in defibrillation/cardioversion threshold, Inability to defibrillate or pace, Inability to interrogate or program due to programmer or device malfunction, Incomplete lead connection with pulse generator, Inhibited therapy including defibrillation and pacing, Inappropriate therapy (for example, shocks and antitachycardia pacing [ATP] where applicable, pacing), Interruption of function due to electrical or magnetic interference, Intolerance to high rate pacing (for example dyspnea or discomfort), Lead abrasion, Lead fracture, Lead insulation damage, Lead migration or lead dislodgement, Loss of device functionality due to component failure, Pulse generator migration, Rise in DFT threshold, Rise in pacing threshold and exit block, Shunting of energy from defibrillation paddles, System failure due to ionizing radiation. Additionally, potential adverse events associated with the implantation of a coronary venous lead system include the following: Allergic reaction to contrast media, Breakage or failure of implant instruments, Prolonged exposure to fluoroscopic radiation, Renal failure from contrast media used to visualize coronary veins. Refer to the User’s Manual for detailed intended use, indications, contraindications, warnings, precautions and potential adverse events.
No potential adverse events have been identified with use of the myMerlinPulse™ application.
™ Indicates a trademark of the Abbott group of companies.
‡ Indicates a third-party trademark, which is property of its respective owner.
Bluetooth and the Bluetooth logo are registered trademarks of Bluetooth SIG, Inc.
© 2024 Abbott. All Rights Reserved.
MAT-2003052 v6.0 | Item approved for Global use.
Consumer Health Data Privacy Policy

How to pair your iphone

How to pair your android